You are here
Species
Estrildidae
EOL Text
Collection Sites: world map showing specimen collection locations for Estrildidae![]()
The estrildid finches are small passerine birds of the Old World tropics and Australasia. They can be classified as the family Estrildidae (weaver-finch), or as a subfamily within the family Passeridae, which strictly defined comprises the Old World sparrows.[1]
They are gregarious and often colonial seed-eaters with short, thick, but pointed bills. They are all similar in structure and habits, but vary widely in plumage colours and patterns.
All the estrildids build large, domed nests and lay 5–10 white eggs. Many species build roost nests. Some of the fire-finches and pytilias are hosts to the brood-parasitic indigobirds and whydahs, respectively.
Most are sensitive to cold and require warm, usually tropical, habitats, although a few have adapted to the cooler climates of southern Australia.
The smallest species of the family is the Shelley's Oliveback (Nesocharis shelleyi) at a mere 8.3 centimetres (3.3 in), although the lightest species is the Black-rumped Waxbill (Estrilda troglodytes) at 6 g (0.21 oz). The largest species is the Java Sparrow (Padda oryzivora), at 17 cm (6.7 in) and 25 g (0.88 oz).
Contents |
Species list
- Antpeckers, genus Parmoptila
- Red-fronted Antpecker, Parmoptila rubrifrons
- Jameson's Antpecker, Parmoptila jamesoni – often included in P. rubrifrons
- Woodhouse's Antpecker, Parmoptila woodhousei
- Nigritas, genus Nigrita
- White-breasted Nigrita, Nigrita fusconota
- Chestnut-breasted Nigrita, Nigrita bicolor
- Pale-fronted Nigrita, Nigrita luteifrons
- Grey-headed Nigrita, Nigrita canicapilla
- Olivebacks, genus Nesocharis
- White-collared Oliveback, Nesocharis ansorgei
- Shelley's Oliveback, Nesocharis shelleyi
- Grey-headed Oliveback, Nesocharis capistrata
- Pytilias, genus Pytilia
- Orange-winged Pytilia, Pytilia afra
- Red-winged Pytilia, Pytilia phoenicoptera
- Red-billed Pytilia, Pytilia lineata
- Green-winged Pytilia, Pytilia melba
- Yellow-winged Pytilia, Pytilia hypogrammica
- Crimsonwings, genus Cryptospiza
- Red-faced Crimsonwing, Cryptospiza reichenovii
- Abyssinian Crimsonwing, Cryptospiza salvadorii
- Dusky Crimsonwing, Cryptospiza jacksoni
- Shelley's Crimsonwing, Cryptospiza shelleyi
- Seedcrackers, genus Pyrenestes
- Crimson Seedcracker, Pyrenestes sanguineus
- Black-bellied Seedcracker, Pyrenestes ostrinus
- Lesser Seedcracker, Pyrenestes minor
- Bluebills, genus Spermophaga
- Grant's Bluebill, Spermophaga poliogenys
- Western Bluebill, Spermophaga haematina
- Red-headed Bluebill, Spermophaga ruficapilla
- Genus Mandingoa
- Green-backed Twinspot, Mandingoa nitidula
- Genus Clytospiza
- Brown Twinspot, Clytospiza monteiri
- Genus Hypargos
- Red-throated Twinspot, Hypargos niveoguttatus
- Pink-throated Twinspot, Hypargos margaritatus
- Genus Euschistospiza
- Dybowski's Twinspot, Euschistospiza dybowskii
- Dusky Twinspot, Euschistospiza cinereovinacea
- Firefinches, genus Lagonosticta
- Bar-breasted Firefinch, Lagonosticta rufopicta
- Brown Firefinch, Lagonosticta nitidula
- Red-billed Firefinch, Lagonosticta senegala
- Black-bellied Firefinch, Lagonosticta rara
- African Firefinch, Lagonosticta rubricata
- Landana Firefinch, Lagonosticta landanae
- Jameson's Firefinch, Lagonosticta rhodopareia
- Mali Firefinch, Lagonosticta virata
- Rock Firefinch, Lagonosticta sanguinodorsalis
- Black-faced Firefinch, Lagonosticta larvata
- Chad Firefinch, Lagonosticta umbrinodorsalis
- Cordon-bleus, genus Uraeginthus
- Blue Waxbill, Uraeginthus angolensis
- Red-cheeked Cordon-bleu, Uraeginthus bengalus
- Blue-capped Cordon-bleu, Uraeginthus cyanocephalus
- Purple Grenadier, Uraeginthus ianthinogaster
- Violet-eared Waxbill, Uraeginthus granatinus
- Genus Coccopygia
- Yellow-bellied Waxbill, Coccopygia quartinia
- Swee Waxbill, Coccopygia melanotis
- Waxbills, genus Estrilda
- Lavender Waxbill, Estrilda caerulescens
- Grey Waxbill, Estrilda perreini
- Cinderella Waxbill, Estrilda thomensis
- Fawn-breasted Waxbill Estrilda paludicola
- Anambra Waxbill, Estrilda poliopareia
- Orange-cheeked Waxbill, Estrilda melpoda
- Arabian Waxbill, Estrilda rufibarba
- Crimson-rumped Waxbill, Estrilda rhodopyga
- Black-rumped Waxbill, Estrilda troglodytes
- Common Waxbill, Estrilda astrild
- Black-lored Waxbill, Estrilda nigriloris
- Black-crowned Waxbill, Estrilda nonnula
- Black-headed Waxbill, Estrilda atricapilla
- Kandt's Waxbill, Estrilda kandti
- Black-faced Waxbill, Estrilda erythronotos
- Black-cheeked Waxbill, Estrilda charmosyna
- Avadavats, genus Amandava
- Red Avadavat, Amandava amandava also known as Red Munia
- Green Avadavat, Amandava formosa
- Orange-breasted Waxbill, Amandava subflava
- Quailfinches, genus Ortygospiza
- Black-chinned Quailfinch, Ortygospiza gabonensis
- Black-faced Quailfinch, Ortygospiza atricollis
- African Quailfinch, Ortygospiza fuscocrissa
- Genus Paludipasser
- Locust Finch, Paludipasser locustella
- Genus Emblema
- Painted Finch, Emblema pictum
- Genus Stagonopleura
- Beautiful Firetail, Stagonopleura bella
- Red-eared Firetail, Stagonopleura oculata
- Diamond Firetail, Stagonopleura guttata
- Genus Oreostruthus
- Mountain Firetail, Oreostruthus fuliginosus
- Genus Neochmia
- Red-browed Finch, Neochmia temporalis
- Crimson Finch, Neochmia phaeton
- Star Finch, Neochmia ruficauda
- Plum-headed Finch, Neochmia modesta
- Genus Taeniopygia
- Zebra Finch, Taeniopygia guttata
- Double-barred Finch, Taeniopygia bichenovii
- Genus Poephila
- Masked Finch, Poephila personata
- Long-tailed Finch, Poephila acuticauda
- Black-throated Finch, Poephila cincta
- Parrotfinches, genus Erythrura
- Tawny-breasted Parrotfinch, Erythrura hyperythra
- Pin-tailed Parrotfinch, Erythrura prasina
- Green-faced Parrotfinch, Erythrura viridifacies
- Tricolored Parrotfinch, Erythrura tricolor
- Blue-faced Parrotfinch, Erythrura trichroa
- Red-eared Parrotfinch, Erythrura coloria
- Papuan Parrotfinch, Erythrura papuana
- Red-throated Parrotfinch, Erythrura psittacea
- Fiji Parrotfinch, Erythrura pealii
- Red-headed Parrotfinch, Erythrura cyaneovirens
- Royal Parrotfinch, Erythrura regia
- Pink-billed Parrotfinch, Erythrura kleinschmidti
- Gouldian Finch, Erythrura gouldiae
- Munias, Mannikins and Silverbills, genus Lonchura
- Madagascar Mannikin, Lonchura nana
- African Silverbill, Lonchura cantans
-
- Indian Silverbill, Lonchura malabarica also known as White-throated Munia
- Grey-headed Silverbill, Lonchura griseicapilla
- Bronze Mannikin, Lonchura cucullata also known as Bronze Munia
- Black-and-white Mannikin, Lonchura bicolor also known as Black-and-white Munia
- Red-backed Mannikin, Lonchura nigriceps also known as Brown-backed Munia
- Magpie Mannikin, Lonchura fringilloides also known as Magpie Munia
- White-rumped Munia, Lonchura striata
- Javan Munia, Lonchura leucogastroides
- Dusky Munia, Lonchura fuscans
- Black-faced Munia, Lonchura molucca
- Black-throated Munia, Lonchura kelaarti also known as Jerdon's Mannikin
-
- Scaly-breasted Munia, Lonchura punctulata also known as Nutmeg Mannikin or Spice Finch
- White-bellied Munia, Lonchura leucogastra
- Streak-headed Munia, Lonchura tristissima
- Chestnut Munia, Lonchura atricapilla
- Tricoloured Munia, Lonchura malacca
- White-capped Munia, Lonchura ferruginosa
- Cream-bellied Munia, Lonchura pallidiventer
-
- Five-coloured Munia, Lonchura quinticolor
- White-headed Munia, Lonchura maja
- Pale-headed Munia, Lonchura pallida
- Great-billed Mannikin, Lonchura grandis
- Grey-banded Mannikin, Lonchura vana
- Grey-crowned Mannikin, Lonchura nevermanni
- Hooded Mannikin, Lonchura spectabilis
- Grey-headed Mannikin, Lonchura caniceps
- Hunstein's Mannikin, Lonchura hunsteini
- Forbes's Mannikin, Lonchura forbesi
- New Hanover Mannikin, Lonchura nigerrima
- Yellow-rumped Mannikin, Lonchura flaviprymna
- Chestnut-breasted Mannikin, Lonchura castaneothorax
-
- Black Mannikin, Lonchura stygia
- Black-breasted Mannikin, Lonchura teerinki
- Western Alpine Mannikin, Lonchura montana
- Eastern Alpine Mannikin, Lonchura monticola
- Buff-bellied Mannikin, Lonchura melaena
- Java Sparrow, Lonchura oryzivora
- Timor Sparrow, Lonchura fuscata
- Genus Heteromunia
- Pictorella Mannikin, Heteromunia pectoralis
- Cut-throats, genus Amadina
- Cut-throat Finch, Amadina fasciata
- Red-headed Finch, Amadina erythrocephala
Evolution
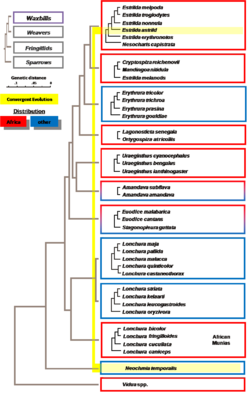
The phylogeography and possible origin of estrildid finches have been studied. The following scheme may be useful to represent an hypothetical origin in India in the last and stronger Himalayas uplift (16.5 million years ago),when monsoon rains regime established in India (see figure). The conclusions from this study[2][3] are:
- Estrildids are a monophyletic group with polytomies that may have started evolving by Middle Miocene Epoch (about 16.5 million years ago)
- This proposed timing is coincidental with the Fringillinae finches’ radiation starting time and also with the biggest Himalayan and Tibetan Plateau uplift, triggered by the Indian tectonic plate strongest collision; this established present day southern Asia monsoon regime and other drastic climatic changes, like a dryer weather in Tibetan Plateau and China deserts.
- The Estrildid finches form a monophyletic group which includes several polytomies and comprises African, Asian and Australian birds.
- The most ancient evolutive group comprises African (African silverbill), Asian (Indian silverbill) and Australian (diamond firetail); this suggests that the whole Estrildids radiation might have originated around India.[2][4][5]
- The African group Nesocharis is grouped with the African gender Estrilda.
- The Gouldian Finch (Erythrura or Chloebia gouldiae) is definitely included within genus Erythrura with the other species.
- The Java sparrow (Padda or Lonchura oryzivora) is a very modified species from genus Lonchura: bigger size than the rest of Lonchura species, and a noticeable and quite different head pattern. It is endemic from Java, Bali, and Bawean Islands, although escapes from captivity can be seen today in other neighboring islands.
- African munias (Spermestes) belong to a genus totally different to Australian and Asian munias.
- The Australian species Red-browed Firetail (Neochmia temporalis), very similar to African common waxbill (Estrilda astrild), is unrelated to it. Their similarities (bill, red brow, etc.) are due to convergent evolution, since their environmental pressures (weather, habitat, feeding) are similar.[5][2]
References
- ^ Christidis L, Boles WE (2008). Systematics and Taxonomy of Australian Birds. Canberra: CSIRO Publishing. p. 177. ISBN 978-0-643-06511-6.
- ^ a b c Arnaiz-Villena,A; Ruiz-del-Valle,V.; Gomez-Prieto,P.; Reguera,R.; Parga-Lozano,C; Serrano-Vela,J.I. (2009). "Estrildinae Finches (Aves, Passeriformes) from Africa, South Asia and Australia: a Molecular Phylogeographic Study"(PDF). The Open Ornithology Journal 2: 29-36. (doi:10.2174/1874453200902010029).
- ^ Arnaiz-Villena, A; Gómez-Prieto P, Ruiz-de-Valle V (2009). "Phylogeography of finches and sparrows". Nova Science Publishers. ISBN 978-1-60741-844--3.
- ^ Sibley CG, Monroe BL (1990). Distribution and Taxonomy of Birds of the World. Yale University Press
- ^ a b Arnaiz-Villena,A; Gomez-Prieto,P.; Serna-Ayala; Ruiz-del-Valle,V. (2009). "Origen de los estríldidos".
| License | http://creativecommons.org/licenses/by-sa/3.0/ |
| Rights holder/Author | Wikipedia |
| Source | http://en.wikipedia.org/w/index.php?title=Estrildid_finch&oldid=547484423 |
The Passeriformes is the largest and most diverse commonly recognized clade of birds. The Passeriformes (or ‘passerine’ birds) are synonymous with what are commonly known as "perching birds"; this group also contains within it a major radiation commonly known as songbirds (oscine Passerines or Passeri). Of the 10,000 or so extant species of birds, over half (~5,300) are perching birds.
Perching birds have a worldwide distribution, with representatives on all continents except Antarctica, and reaching their greatest diversity in the tropics. Body sizes of passerines vary from about 1.4 kg in northern populations of Ravens (Corvus corax) to just a few grams. Perching birds include some of the most colorful and mysterious of all birds, such as birds of paradise from New Guinea and the bright orange Cock of the Rock from tropical South America. Because of their high diversity, generally small body size and relative ease of observation, collection and field study, perching birds have historically attracted the attention of a wide range of descriptive and experimental biologists, including systematists, behavioral ecologists, and evolutionary biologists.
| License | http://creativecommons.org/licenses/by-nc/3.0/ |
| Rights holder/Author | Cyndy Parr, Cyndy Parr |
| Source | http://tolweb.org/Passeriformes/15868/2013.02.06 |
In Great Britain and/or Ireland:
Animal / parasite / ectoparasite
imago of Crataerina pallida ectoparasitises mainly adult of Passeriformes
Other: minor host/prey
Animal / parasite / ectoparasite
imago of Ornithomya avicularia ectoparasitises Passeriformes
Animal / parasite / ectoparasite
imago of Ornithomya fringillina ectoparasitises Passeriformes
| License | http://creativecommons.org/licenses/by-nc-sa/3.0/ |
| Rights holder/Author | BioImages, BioImages - the Virtual Fieldguide (UK) |
| Source | http://www.bioimages.org.uk/html/Passeriformes.htm |
Passeriformes (song birds) is prey of:
Circus
Asio
Based on studies in:
USA: California (Marine)
This list may not be complete but is based on published studies.
- R. F. Johnston, Predation by short-eared owls on a Salicornia salt marsh, Wilson Bull. 68(2):91-102, from p. 99 (1956).
| License | http://creativecommons.org/licenses/by/3.0/ |
| Rights holder/Author | Cynthia Sims Parr, Joel Sachs, SPIRE |
| Source | http://spire.umbc.edu/fwc/ |
Passeriformes (song birds) preys on:
Plantae
invertebrates
marine invertebrates
Insecta
Prokelisia
Orchelimum
Araneae
Based on studies in:
USA: California (Marine)
USA: Massachusetts, Cape Ann (Marine)
USA: Georgia (Marine)
This list may not be complete but is based on published studies.
- R. W. Dexter, The marine communities of a tidal inlet at Cape Ann, Massachusetts: a study in bio-ecology, Ecol. Monogr. 17:263-294, from p. 287 (1947).
- R. W. Dexter, The marine communities of a tidal inlet at Cape Ann, Massachusetts: a study in bio-ecology, Ecol. Monogr. 17:263-294, from p. 288 (1947).
- J. M. Teal, Energy flow in the salt marsh ecosystem of Georgia, Ecology 43(4):614-624, from p. 616 (1962).
- R. F. Johnston, Predation by short-eared owls on a Salicornia salt marsh, Wilson Bull. 68(2):91-102, from p. 99 (1956).
| License | http://creativecommons.org/licenses/by/3.0/ |
| Rights holder/Author | Cynthia Sims Parr, Joel Sachs, SPIRE |
| Source | http://spire.umbc.edu/fwc/ |
It is extremely difficult to generalize about any of the behaviors or nesting habits of passerines, because as a group they are so diverse. Perching birds exhibit a bewildering array of plumages and colors derived from diverse keratin structures as well as ingested pigments, such as carotenoids (Gray, 1996). Many passerines, such as some Old World Flycatchers (Muscicapidae) and African Widowbirds (Viduinae) have extremely long tail feathers or highly modified plumes (Birds of Paradise: Paradisaeidae) used in courtship displays. Several groups such as the Wattlebirds of New Zealand (Callaeidae) and Honeyeaters (Meliphagidae) have fleshy, bright blue, red or yellow wattles on the face and neck. Perching birds build their nests generally out of sticks or grass on the ground, in trees, and in the case of Dippers (Cinclidae) in the banks of fast-flowing rivers. Many passerines migrate from their nesting grounds in the Nearctic and Palearctic to more equatorial regions, or from southern temperate regions north to the tropics. Parental care by both sexes is common in passerines, although in some highly dimorphic and predominantly lekking groups, such as manakins (Prum, 1994) and birds of paradise (Diamond, 1986), females alone provide for young and build the nest. Cooperative breeding, in which young birds delay breeding and assist other individuals (often parents) in raising young and defending the territory, is common in several passerine groups, such as Australian fairy wrens (Maluridae) and New World Jays (Corvidae; Brown, 1987; Edwards and Naeem, 1993). Some of the most elaborate singers in the bird world are passerines (Kroodsma and Miller, 1996). Some passerine birds are poisonous to the touch and are avoided as prey by indigenous peoples (Dumbacher et al., 1992).
- Brown, J. L. (1987). Helping and Communal Breeding in Birds. In Monographs in Behavioral Ecolog. Princeton, N.J.: Princeton University Press.
- Diamond, J. (1986). Biology of birds of paradise and bowerbirds. Annual Review of Ecology and Systematics 17, 17-37.
- Dumbacher, J. P., Beehler, B. M., Spande, T. F., Garraffo, H. M. and Daly, J. W. (1992). Homobatrachotoxin in the genus Pitohui : chemical defence in birds? Science 258, 799-801.
- Edwards, S. V. and Naeem, S. (1993). The phylogenetic component of cooperative breeding in perching birds. The American Naturalist 141, 754-789.
- Gray, D. A. (1996). Carotenoids and sexual dichromatism in North American passerine birds. The American Naturalist 148, 453-480.
- Kroodsma, D. E. and Miller, E. H. (1996). Ecology and Evolution of Acoustic Communication in Birds. Ithaca, NY: Cornell University Press.
- Prum, R. O. (1994). Phylogenetic analysis of alternative social behavior in the manakins (Aves: Pipridae). Evolution 48, 1657-1665.
| License | http://creativecommons.org/licenses/by-nc/3.0/ |
| Rights holder/Author | Cyndy Parr, Cyndy Parr |
| Source | http://tolweb.org/Passeriformes/15868/2013.02.06 |
Species diversity: The tradition of recognizing perching birds (Passeriformes) as the most diverse and rapidly radiating clade has been questioned because there are few obvious “key innovations” that should cause systematists to recognize Passeriformes over any other arbitrarily larger or smaller monophyletic group within birds (Raikow, 1986). One point that has been missed in debates on this issue is that the branch leading to the songbirds (oscines), a group comprising 80% of extant perching birds, is the longest internal branch on the DNA hybridization tree produced by Sibley and Ahlquist (1990). This branch has also been one of the few to be well resolved in applications of mtDNA sequences to higher level questions in birds, presumably because it is long. Given the large number of clades that will require names under phylogenetic taxonomy, perhaps the length of branches leading to particular clades should be one criterion whereby systematists decide which of the many clades to name.
Origin and biogeography of passerines: The temporal and geographic origin of passerine birds is obscure. Traditionally the group was thought to have originated in the Tertiary, at about the same time as extant orders of mammals. Some recent workers favor a later, Eocene origin (Feduccia, 1995; Wilson, 1989), but the DNA -DNA hybridization data again favors an earlier origin (Sibley and Ahlquist, 1990). Recently some of the oldest oscine fossils have been uncovered in Queensland, Australia (Boles, 1995); this and other paleobiogeographical data suggest that passerines may have in fact originated in the Southern hemisphere (Olson, 1989).
- Boles, W. E. (1995). The world's oldest songbird. Nature 374, 21-22.
- Feduccia, A. (1995). Explosive radiation in Tertiary birds and mammals. Science 267, 637-638.
- Olson, S. L. (1989). Aspects of global avifaunal dynamics during the Cenozoic. In Proceedings of the XIX International Ornithological Congress, vol. 29, pp. 2023-2029. Christchurch, New Zealand.
- Raikow, R. J. (1986). Why are there so many kinds of passerine birds? Systematic Zoology 35, 255-259.
- Sibley, C. G. and Ahlquist, J. E. (1990). Phylogeny and Classification of Birds. New Haven, CT: Yale University Press.
- Wilson, A. C. (1989). Time scale for bird evolution. Proceedings of the XIX International Ornithological Congress 19, 1912-1917.
| License | http://creativecommons.org/licenses/by/3.0/ |
| Rights holder/Author | Cyndy Parr, Cyndy Parr |
| Source | http://tolweb.org/Passeriformes/15868/2013.02.06 |
Historically, it is generally agreed that the Passeriformes constitute a monophyletic group. Raikow (1982) established this monophyly in an explicitly phylogeneticcontext. He noted that Passeriformes possess a suite of distinguishing characteristics, including a unique sperm morphology, a distinctive morphology of the bony palate, a simple yet functionally diverse foot with three toes forward and one (the hallux) oriented backwards, and a distinctive fore- (wing) and hindlimb musculature. There are few if any species which pose problems for avian systematists as to whether they are or are not passerines. Most of the controversy lies in relationships within the clade.
The sister group of the Passeriformes is not so much hotly contested as it is poorly resolved by existing data sets. Traditionally, Passeriformes have been considered closely related to a large group known as the “higher non-Passerines”. These include a number of clades such as cuckoos (Cuculiformes), hornbills, kingfishers and related lineages (Coraciiformes), and woodpeckers and relatives (Piciformes). Many of these groups possess a zygodactyl foot, a condition in which two toes point forward and two point backward. The sister relationship of Passeriformes to woodpeckers, the hornbill group and allies is reflected in Joel Cracraft’s phylogenetic hypothesis for major groups of birds based on cladistic interpretation of morphological and molecular characters (Cracraft, 1988). However, in the other major classification bearing on the relationships of perching birds, that based on DNA-DNA hybridization, Passeriformes appear as the sister group to a large, diverse group containing pigeons and doves (Columbiformes), cranes and rails (Gruiformes) and storks (Ciconiiformes)! These latter three groups share few obvious morphological characteristics with Passeriformes. However, the DNA hybridization tree links Passeriformes with these groups at a very deep level in the tree, rendering this result tenuous. A recent study of nuclear DNA sequences by Hackett et al. (2008) finds Psittaciformes (parrots) to be the sister group of passerines, with Falconidae (falcons) also close. Clearly, more work on the sister-group relationship of Passeriformes is needed, since this relationship will be the basis of any study seeking to identify whether or not Passeriformes are a particularly diverse group (e.g., Nee et al. 1992).
- Cracraft, J. (1988). The major clades of birds. In The Phylogeny and Classification of the Tetrapods, Volume 1: Amphibians, Reptiles, Birds, vol. 35A (ed. M.J.Benton), pp. 339-361. Oxford: Clarendon Press.
- Hackett, S. J., Kimball, R. T., Reddy, S., Bowie, R. C. K., Braun, E. L., Braun, M. J., Chojnowski, J. L., Cox, W. A., Han, K.-L., Harshman, J., Huddleston, C. J., Marks, B. D., Miglia, K. J., Moore, W. A., Sheldon, F. H.,
- Nee, S., Mooers, A., and Harvey, P. H. (1992). Tempo and mode of evolution revealed from molecular phylogenies. Proceedings of the National Academy of Sciences (USA) 89, 8322-8326.
- Raikow, R. J. (1982). Monophyly of the Passeriformes: test of a phylogenetic hypothesis. Auk 99, 431-455.
| License | http://creativecommons.org/licenses/by-nc/3.0/ |
| Rights holder/Author | Cyndy Parr, Cyndy Parr |
| Source | http://tolweb.org/Passeriformes/15868/2013.02.06 |